Reductive Amination
(NaBH4)
Examples:
Example 1
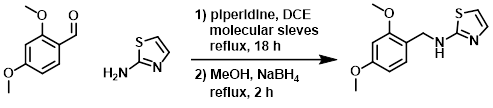
The amine (15.1 g, 150 mmol), aldehyde (25 g, 150 mmol), and piperidine (150 mg, 1.76 mmol) in DCE (500 mL) were refluxed over molecular sieves for 18 h. The molecular sieves were filtered away and the reaction mixture was diluted with MeOH (300 mL). NaBH4 (25 g, 662 mmol) was added portionwise and the mixture was refluxed for 2 h. The mixture was cooled, quenched with H2O, and the org solvents were removed in vacuo. The resulting mixture was extracted with EtOAc. The combined organics were extracted with 2N HCl. The aq layer was basified with K2CO3 and re-extracted with EtOAc, dried (Na2SO4), and concentrated. The resulting material was purified by column chromatography (10% MeOH/DCM) to provide the product. [24 g, 96 mmol]
[Patent Reference: WO2010035166, page 66, ![]() (3.2 MB)]
(3.2 MB)]
Example 2
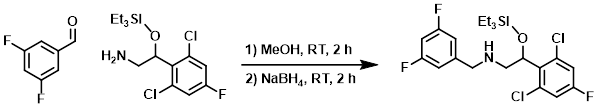
To a solution of the amine (30.0 g, 88.7 mmol) in MeOH (200 mL) was added the aldehyde (12.6 g, 88.7 mmol). The mixture was stirred at RT for 2 h. Upon completion of imine formation (monitored by TLC), NaBH4 (4.9 g, 133.1 mmol) was added portionwise at 0 C. The mixture was warmed to RT and stirred for 2 h, after which time it was quenched with H2O (100 mL) and extracted with EtOAc (3 x 100 mL). The combined organics were washed with brine (2 x 75 mL), dried (Na2SO4), and concentrated. The resulting material was purified by silica gel column chromatography (10% EtOAc/hexane) to provide the product as a colorless gum. [30.0 g, 70%]
[Patent Reference: WO2015129926, page 74, ![]() (21.5 MB)]
(21.5 MB)]
Example 3
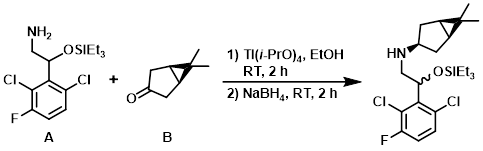
The amine (A) (0.493 g, 1.457 mmol) and the ketone (B) (0.181 g, 1.457 mmol) were combined in dry EtOH (7 mL) under N2 at RT and Ti(i-PrO)4 (0.86 mL, 2.91 mmol) was added. The reaction mixture was stirred at RT for 2 h, after which time NaBH4 (0.083 g, 2.186 mmol) was added. After 2 h, the mixture was quenched with sat aq NH4Cl (3 mL) and then basified with sat aq NaHCO3. The EtOH was removed in vacuo, and the solution was diluted with H2O and EtOAc. Celite was added and the mixture was vigorously mixed for 15 min. The mixture was filtered through a pad of celite. The aq layer was extracted with EtOAc and the combined organics were washed with brine, dried (Na2SO4), and concentrated to give a yellow oil. The crude material was purified by silica gel column chromatography (0-10% EtOAc/heptane) to provide the product as a colorless oil. [414 mg, 63.6%]
[Patent Reference: WO2015129926, page 176, ![]() (21.5 MB)]
(21.5 MB)]
